軟部肉腫のGEM+DTXレジメン申請資料
軟部肉腫 レジメン 腫瘍内科
2024年2月26日の厚労省通知で進行軟部肉腫に対してGEM+DTXも認められるという情報が出ていたので、それのレジメン申請用の資料です。
https://www.jges.net/wp-content/uploads/2024/03/e61aad662b27e9417ea75179604eb8ac.pdf
GEMをday1,8に900mg/m2、DTXをday8に70mg/m2に投与し、これを3週回し。
そのレジメン申請資料としては、以下の文献が使えそうです。
日本の多施設後方視的報告

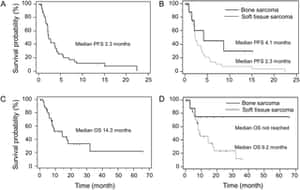
Background Bone and soft tissue sarcomas (BSTS) are rare malignant tumors. Recently, the combination of gemcitabine and docetaxel (GD) was shown to have activity as second-line setting in BSTS. However, the efficacy as first-line and adjuvant settings and precise profiles of adverse events in Japanese patients are not known yet. In the present study, the feasibility and efficacy of GD in patients with BSTS were investigated. Methods Patients with BSTS treated with GD in our institutions were retrospectively analyzed. Information regarding clinical features, adverse events, and outcome was collected and statistically studied. Factors related to survival were analyzed using log-rank test and Cox proportional hazard regression method. Results A total of 134 patients were analyzed. GD was carried out as adjuvant setting in 9, first-line in 23, second-line in 56, and third-or-greater line in 46 patients. The response rate (RR) for all patients was 9.7%. RR for the patients treated as adjuvant or first-line setting was 18.8%, whereas that as second-or-greater line was 6.9%. The median progression-free survival (PFS) and overall survival (OS) of all patients were 4.8 (95% CI 3.5?6.1) and 16.4 (95% CI 9.8?22.9) months, respectively. Survival tended to be better in the patients treated as first-line than in those treated as second-or-greater line. Multivariate analysis demonstrated that history of prior chemotherapy (p?=?0.046) and response to GD (p?=?0.009) was significantly associated with PFS and OS, respectively. The leucopenia and neutropenia were the most frequent adverse events, and grade 3 or 4 leucopenia and neutropenia were observed in 69.4 and 72.4% of the patients. Grade 2 or 3 pneumonitis was observed in one (0.7%) and four (3.0%) patients, respectively. All the patients with pneumonitis had experienced prior chemotherapy and/or radiotherapy. Conclusions GD used as both first- and second/later line is effective chemotherapy for a proportion of patients with advanced BSTS. Higher response rate and better outcome was achieved in chemotherapy-na�ve patients. This regimen is associated with high incidence of severe hematological toxicity, as well as the risk of severe pneumonitis, especially in pre-treated patients. GD is promising for further analysis by phase III study for the patients with BSTS.
https://wjso.biomedcentral.com/articles/10.1186/s12957-016-1059-2
合計134名の患者が分析され、GEM+DTX療法は9人に対して術後補助療法的設定で、23人に対して一次治療で、56人に対して二次治療で、46人に対して三次以上の治療で行われました。全患者の応答率(RR)は9.7%で、一次治療または補助治療で治療された患者のORRは18.8%と高いものの、二次以上の治療で治療された患者のORRは6.9%と低い傾向でした。
全患者の中央無進行生存期間(PFS)と全生存期間(OS)はそれぞれ4.8ヶ月(95%CI 3.5–6.1)と16.4ヶ月(95%CI 9.8–22.9)でした。結論として、一次治療および二次/以降の治療として使用されたGEM+DTX療法は、進行した骨および軟部組織肉腫の患者の一部に対して有効な化学療法であり、とくに初回治療例ではより高い応答率と良好な成果が得られました。
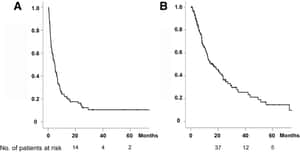
日本の単施設報告

Background Combination therapy with gemcitabine and docetaxel has been reported to be a good therapeutic strategy for patients with soft tissue sarcoma. The aim of the present study was to analyze the efficacy and toxicity of gemcitabine with docetaxel in Japanese patients with advanced bone and soft tissue sarcoma. Patients and methods We retrospectively analyzed the effect of gemcitabine and docetaxel therapy on overall response, progression-free survival, overall survival, and toxicity in 42 patients with bone or soft tissue sarcoma who had received the therapy between October 2006 and September 2015, at Tohoku University Hospital. Results The median age was 55 years; 23 patients were men, and 19 were women. Eight had bone sarcoma and 34 had soft tissue sarcoma. Forty patients (95%) had previously been treated with one or more chemotherapeutic regimens. The overall response rate was 6.9% and the disease control rate was 55%. The median progression-free survival was 2.3 months and the median overall survival was 14.3 months. Grade 3 or more neutropenia and febrile neutropenia were observed in 74% and 4.8% of all patients, respectively. Conclusion The response rate was lower and myelosuppression was more frequently observed than in other previous reports. On the other hand, most of toxicities were enough manageable. In addition, some patients had long survival with a good response. Our study supports the notion that gemcitabine and docetaxel therapy is a good therapeutic option for treating patients with advanced soft tissue sarcoma as well as bone sarcoma, also in Asian populations.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0176972
2006年10月から2015年9月にかけて、東北大学病院でこの療法を受けた骨または軟部肉腫の患者42人のデータを後ろ向きに分析し、患者は42人(男性23人、女性19人)が含まれており、95%の患者が既治療例でした。骨肉腫の患者は8人、軟部肉腫の患者は34人でした。全体の応答率は6.9%、疾患コントロール率は55%でした。中央無進行生存期間は2.3ヶ月、中央全生存期間は14.3ヶ月でした。3度以上の好中球減少症と発熱性好中球減少症は、それぞれ全患者の74%と4.8%に観察されました。
もともと希少がんであり、前向きな臨床試験ではなく後方視的データなどから正規の承認ではなく保険審査上の特例として認められたということに注意が必要です。しかし、治療手段が乏しかった患者層に対してこれらの治療選択肢が提示されたことは非常に貴重なことです。
この記事に対するコメント
このページには、まだコメントはありません。
更新日:2024-03-24 閲覧数:870 views.