TMB高値の固形がんに関するメモ
2019.11.13初稿
2019.11.15一部追記
2020.04.21一部追記
はじめに
がんゲノム医療とがん遺伝子パネル検査の普及に伴ってMSIが治療選択に関わるバイオマーカーの一つとみなされるようになり、また臓器にとらわれないMSI-H固形がんという疾患概念も一般的になってきました。
悪性腫瘍はゲノム異常の蓄積によって引き起こされますが、非常に少数だが致命的なdriver変異によって引き起こされる悪性腫瘍がある一方で、雑多な遺伝子変異の総数が非常に多いhypermutatedな悪性腫瘍もあります。hypermutated固形がんと呼ぶべきかTMB高値固形がんと呼ぶべきか、指すところはほぼ同じですが、ここではTMBとして述べることにします。なお、TMB-highとMSI-Hは重複する特性は多いものの別概念であることに注意が必要です。
主に自分のメモ書きでもあるので雑多な内容ですが、逐次追記します(おそらく)。
TMBはがん種により異なる
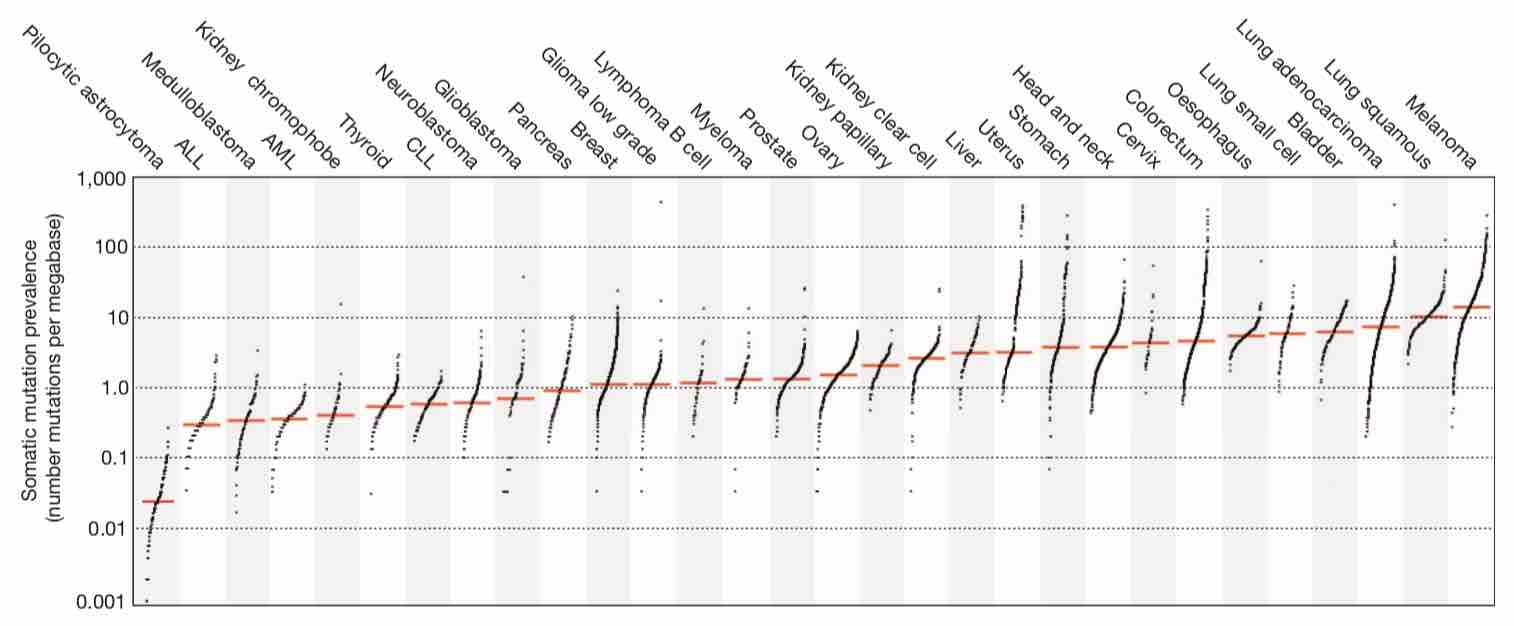
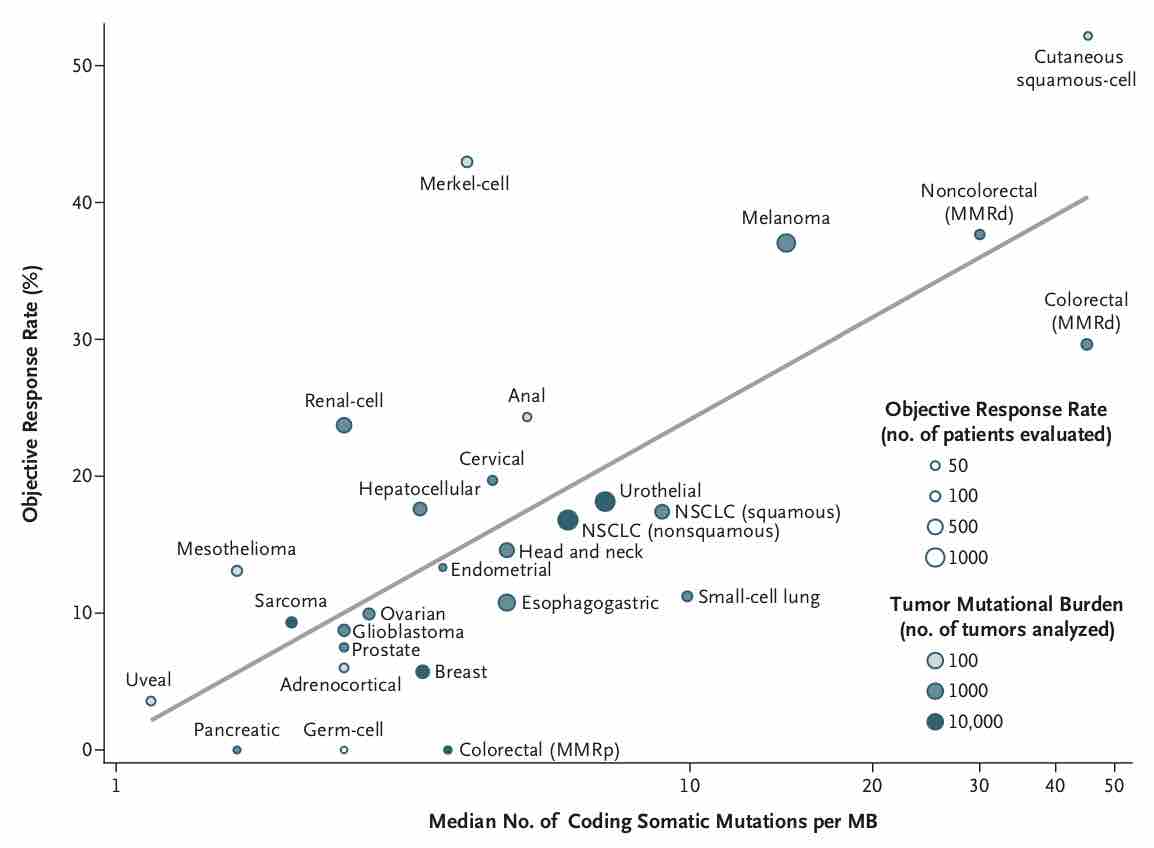
TMBはがん種によって大きく異なることが報告されています。特に悪性黒色腫や非小細胞肺癌などでTMBが高いことが知られていますが、これらはICIの有効性が比較的早期から認められてきたがん種でもあります。
An analysis of mutations from over 7,000 cancers of diverse origins reveals the diversity of mutational processes underlying the development of cancer; more than 20 distinct mutational signatures are described, some of which are present in many cancer types, notably a signature attributed to the APOBEC family of cytidine deaminases, whereas others are specific to individual tumour types.
https://www.nature.com/articles/nature12477
大腸癌は全体としてはTMBが非常に多いがん種ではありませんが、大腸癌の中に一部TMBが非常に高い集団があり、これはMSI-H大腸癌であったりPOLE大腸癌であったりします。一部に、生殖細胞系列にDNAのミスマッチ修復の機能異常を持つLynch症候群などを含みます。
A large-scale analysis of hypermutation in human cancers provides insights into tumor
evolution dynamics and identifies clinically actionable mutation signatures.
https://www.cell.com/cell/fulltext/S0092-8674(17)31142-X
発がん因子、治療歴や進行度によっても生じる差異
MSK-IMPACT is a clinical sequencing platform able to detect genomic mutations, copy number alterations and structural variants in a panel of cancer-related genes. This assay is implemented prospectively to inform patient enrollment in genomically matched clinical trials at Memorial Sloan Kettering Cancer Center (MSKCC). Sequencing results of tumor and matched normal tissue from a cohort of >10,000 patients with detailed clinical annotation provide an overview of the genomic landscape of advanced solid cancers and bring new insights into molecularly guided cancer therapy.
https://www.nature.com/articles/nm.4333
10000人のゲノムの網羅的解析のデータを集めた研究から、同じがん種であっても紫外線や喫煙などの変異源にさらされたことによって生じたがんの方がTMBが高いという報告もあります。一方で白血病や軟部腫瘍や小児がんなどでは少数だが重篤な影響を及ぼす変異によって発癌していることがしばしばあり、TMBは低い傾向にあります。
またテモゾロミドなど細胞障害性化学療法を受けた患者の腫瘍ではTMBが上昇します。化学療法を受けるなかで様々な遺伝子変異が蓄積することで耐性を獲得した細胞が選択されてゆくことでTMBが高くなる方向への誘導が働くことなどが推測されます。
転移巣と原発巣
非小細胞肺癌の原発巣と転移巣のTMBの検討から、もともと腺癌は扁平上皮癌よりTMBが高めだが、腺癌の中でも転移巣は原発巣でTMBが高い傾向があり、脳単位でそれが最も高くなるという報告があります(脳転移するとTMBが高くなるのか、TMBが高いから脳転移するのかはわからない)。

PURPOSE Tumor mutational burden (TMB) is a developing biomarker in non–small-cell lung cancer (NSCLC). Little is known regarding differences between TMB and sample location, histology, or other biomarkers. METHODS A total of 3,424 unmatched NSCLC samples, including 2,351 lung adenocarcinomas (LUADs) and 1,073 lung squamous cell carcinomas (LUSCs), underwent profiling, including next-generation sequencing of 592 cancer-related genes, programmed death ligand 1 immunohistochemistry, and TMB. The rate TMB of 10 mutations per megabase (Mb) or greater was compared between primary and metastatic LUAD and LUSC. Molecular alteration frequency was compared at a cutoff of 10 mutations/Mb. RESULTS LUAD metastases were more likely to have a TMB of 10 mutations/Mb or greater compared with primary LUADs (38% v 25%; P < .001), and this difference was most pronounced with brain metastases (61% v 35% for other metastases; P < .001). The median TMB for LUAD brain metastases was 13 mutations/Mb compared with six mutations/Mb for primary LUADs. Variability existed for other LUAD metastasis sites, with adrenal metastases most likely to meet the cutoff of 10 mutations/Mb (51%) and bone metastases least likely to meet the cutoff (19%). TMB was more commonly 10 mutations/Mb or greater for LUSC primary tumors than for LUAD primary tumors (35% v 25%, respectively; P < .001). LUSC metastases were more likely to have a TMB of 10 mutations/Mb or greater than LUSC primary tumors. Poorly differentiated disease was more likely have a TMB of 10 mutations/Mb or greater when stratified by histology and primary tumor or metastasis. Site-specific molecular differences existed at this TMB cutoff including programmed death ligand 1 positivity and STK11 and KRAS mutation rate. CONCLUSION TMB is a site-specific biomarker in NSCLC with important spatial and histologic differences. TMB is more frequently 10 mutations/Mb or greater in LUAD and LUSC metastases and highest in LUAD brain metastases. Along this TMB cutoff, clinically informative distinctions exist in other tumor profiling characteristics. Further investigation is needed to expand on these findings.
https://ascopubs.org/doi/10.1200/PO.18.00376
変異のタイプによる影響
TMBは変異塩基数を単純にカウントした数ですが、単純に1塩基が置換されたりコドン枠に変化がないinframe-indel(3の倍数の塩基数の欠失挿入)に比べると、frameshiftをきたすindel変異の多さのほうが、ICIの有効性に大きな影響を与えるという報告もあります。
Cancer Research UK, UK National Institute for Health Research (NIHR) at the Royal Marsden Hospital National Health Service Foundation Trust, Institute of Cancer Research and University College London Hospitals Biomedical Research Centres, the UK Medical Research Council, the Rosetrees Trust, Novo No …
https://www.ncbi.nlm.nih.gov/pubmed/28694034
TMBとMSIの関係
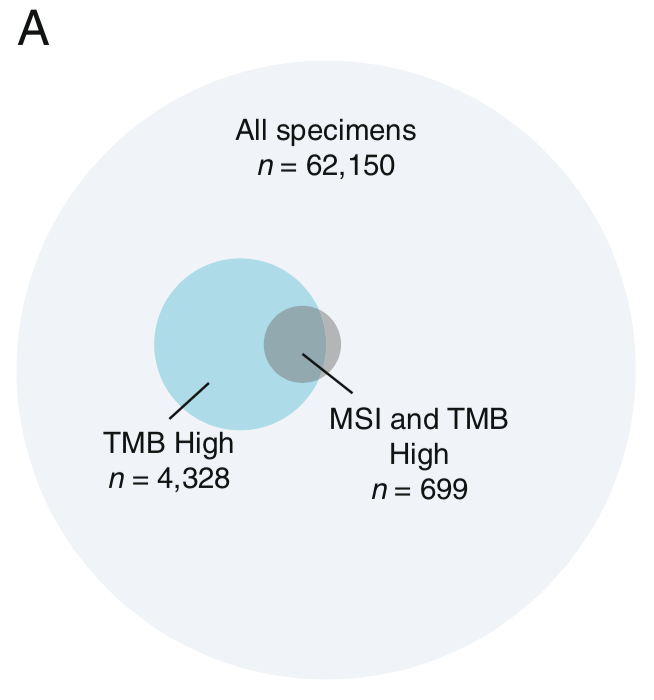
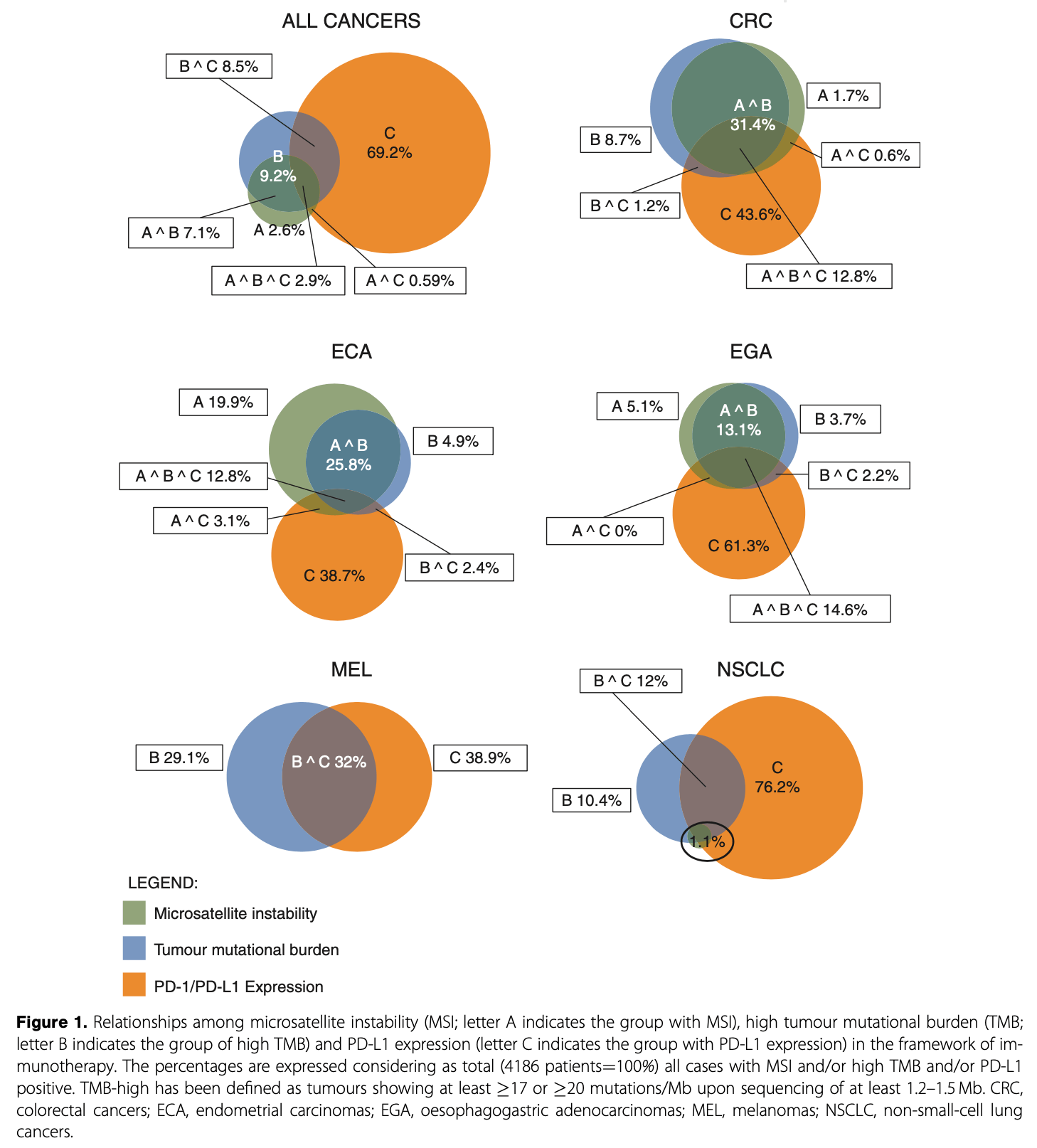
この10万人ゲノム解析の報告によると、mutation burdenが評価できた62150人のうちTMBが4328人でMSIかつTMB-Hは699人。言い換えれば、MSI-Hのうち83%がTMB-HであるがTMB-HのうちMSI-Hは16%しかいません。MSI-HならTMBが高いというのはかなり言えそうですが、TMBが高ければMSI-Hであるというのは若干難しいのではないかという値です。
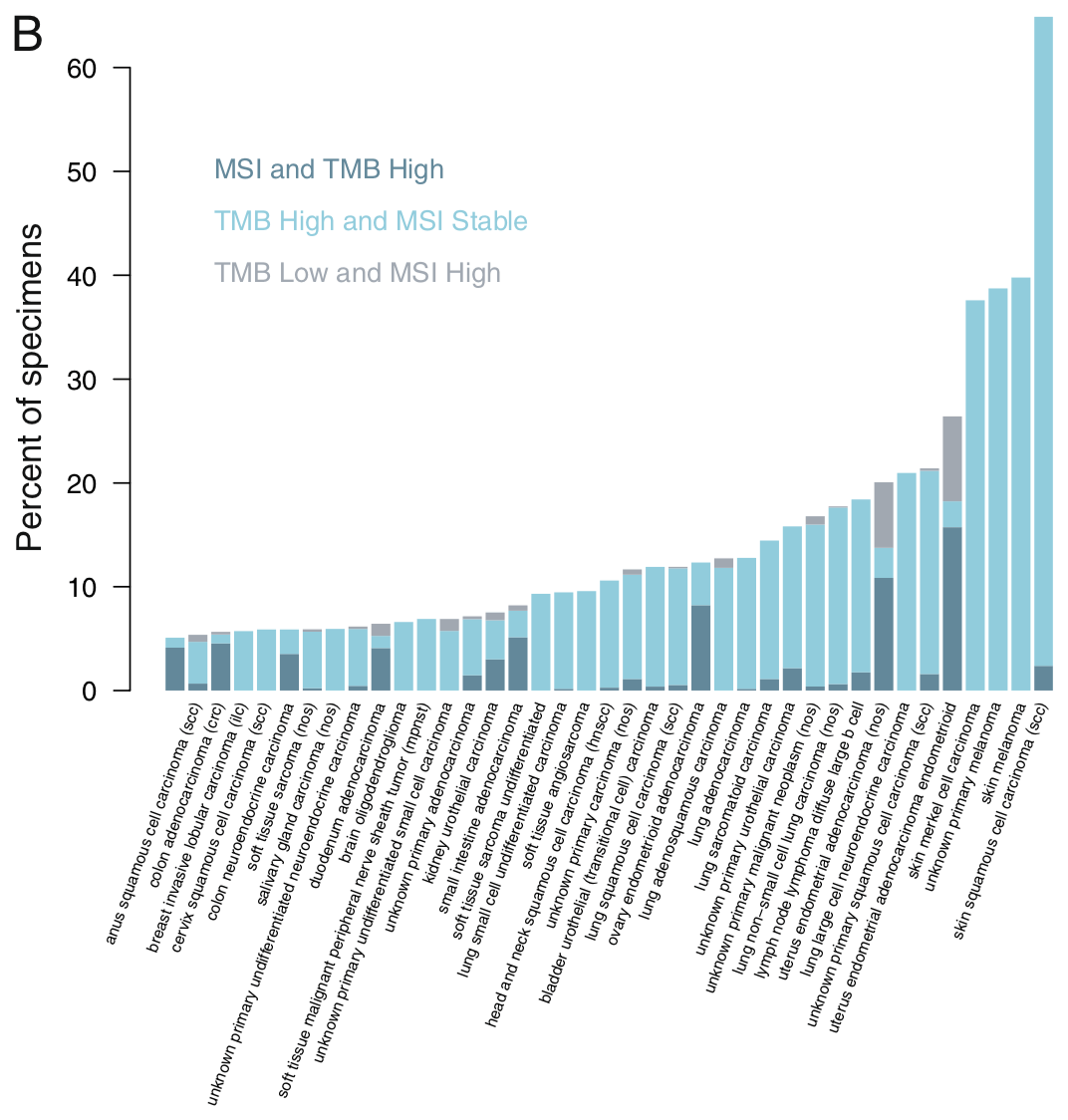
TMB-HとMSI-Hの重なりは消化管癌や子宮体癌ではこのデータより高めのようでもあります。一方で肺癌ではTMBとMSI-Hの相関は乏しくなります。そして悪性黒色腫はこの棒グラフの右から2番目と3番目、いずれも薄水色のバーが高く伸びていて濃い青色はありませんので、TMBは極めて高いのですがMSI-Hはほぼ見られないということになります。
These results show that a CGP assay targeting ~1.1 Mb of coding genome can accurately assess TMB compared with sequencing the whole exome. Using this method, we find that many disease types have a substantial portion of patients with high TMB who might benefit from immunotherapy. Finally, we identif …
https://www.ncbi.nlm.nih.gov/pubmed/28420421
ESMOの推奨の文献*1に載った下の図で言えば食道癌や胃癌ではMSIとTMBは相関が強そうなものの肺癌はそうでもないようです。
Cancers with a defective DNA mismatch repair (dMMR) system contain thousands of mutations
most frequently located in monomorphic microsatellites and are thereby defined as
having microsatellite instability (MSI). Therefore, MSI is a marker of dMMR. MSI/dMMR
can be identified using immunohistochemistry to detect loss of MMR proteins and/or
molecular tests to show microsatellite alterations. Together with tumour mutational
burden (TMB) and PD-1/PD-L1 expression, it plays a role as a predictive biomarker
for immunotherapy.
https://www.annalsofoncology.org/article/S0923-7534(19)31269-4/fulltext
MSI-H陽性腫瘍はTMBが高い
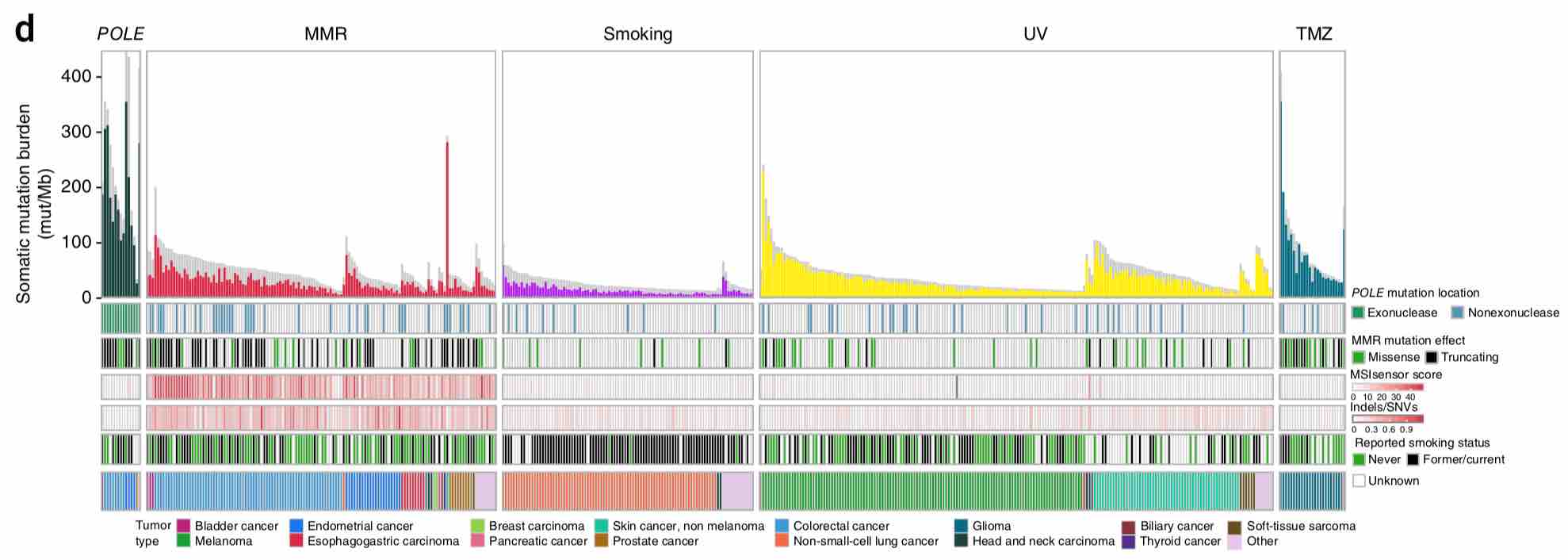
そして、臓器を問わずにTMBが高くhypermutatedながんを来しやすいのが、ミスマッチ修復に異常をきたした悪性腫瘍です。MSH2、MLH1、MSH6、PMS2などのDNAのミスマッチ修復に関連する遺伝子に異常がある場合はDNAに生じた傷が修復されにくくなるため遺伝子異常が蓄積しやすくなり、TMBが高くなります。
パネル検査とTMB
パネル検査やリキッドバイオプシーで推定するTMB
パネル検査は全エクソンあるいは全ゲノムを読んでいるわけではありません。ヒトは2万ほどの遺伝子を持ちますが、パネル検査で読んでいるのはせいぜい100〜300遺伝子ほどに過ぎません。この狭い読み出し範囲からDNA全体の変異数を算出するために各パネルはいろいろな計算式を設定していますが、厳密な意味でのTMBとは異なることに注意が必要です。
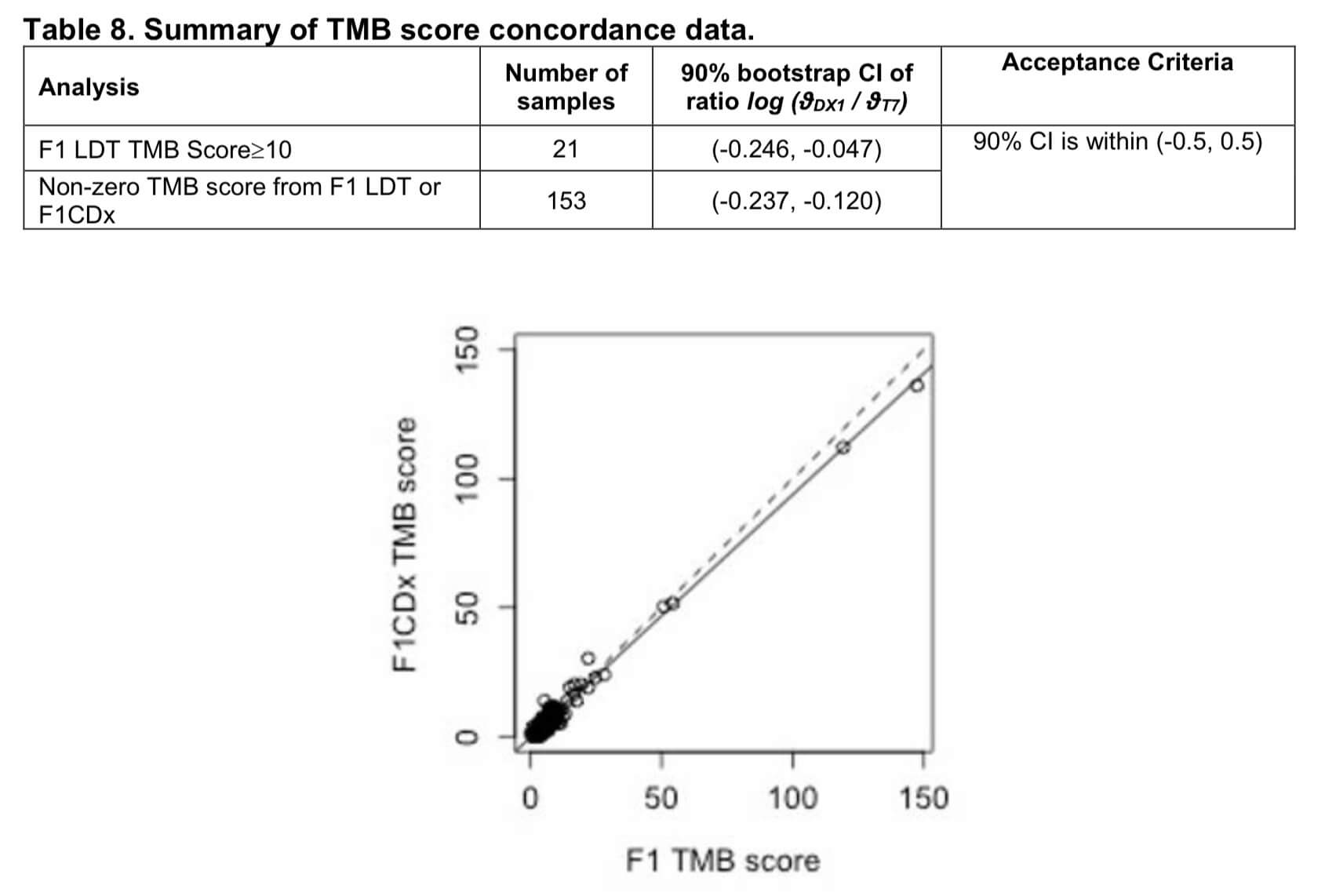
FoundationOne CDxの場合はTMBではなく「TMBスコア」として、変異アレル頻度が5%以上のsynonymousおよびnonsynonymousの変異総数をmut/Mbで表示しています(同様にMSI判定も5箇所のマイクロサテライトに対するPCR法を用いる現行のコンパニオン診断薬での判定と違って、95のイントロンのホモポリマーの繰返し配列の長さのばらつきを解析してMSIスコアを算出するという方法をとっています)。
なお、病的でないSNPsなどの遺伝子多型の影響を排除するために、この過程でdbSNPやExACなどのデータベースに収録されているgermline polymorphismを除外する処理を行っているようです。FoundationOne CDxの資料によると、これはF1LDT TMB測定キットで算出したTMBと高い一致率を誇るとなっています*2。
FoundationOne®CDx Technical Information
https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019C.pdf
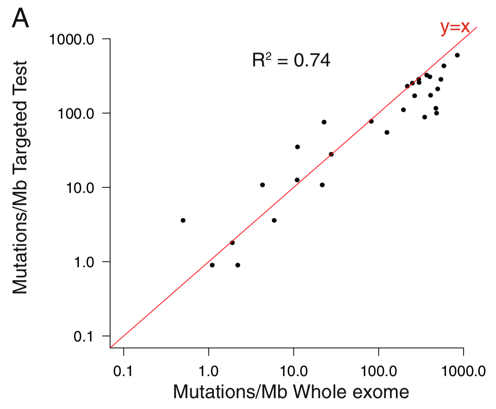
これに関する研究としては、前述の10万人ゲノム解析の研究ではパネル検査と全エクソンシークエンスのMb(100万塩基)あたりの変異数の相関について検討しています。R2=0.74である程度の相関はありそうで、とくにmutation burdenが高めの部分では非常に高い相関性があると言えそうです*3。一方で、7000人以上の臨床検体で3種類のターゲットシークエンス法によるパネル検査と全エクソンシークエンスでのTMBを比較した検討では、パネル検査の方がTMBが高めに算出されるという報告もあります*4。
TMBのカットオフ値
TMBのカットオフ値については統一されたものはありません。同じdMMR(あるいはTMB-high)に対するニボルマブのCHECKMATE試験でも、TMBのカットオフ値は9/Mbから16/Mbまであり、測定方法も標準化されていません。しかし今後は統一された基準を設定しようという動きがあります。
Annals of Oncology, the journal of the European Society for Medical Oncology and the Japanese Society of Medical Oncology, provides rapid and...
https://academic.oup.com/annonc/article/30/1/44/5160130
日本で保険適用となっている2つのパネル検査では、当初はNCCオンコパネルはリード領域全体での変異頻度10mut/Mbをカットオフ値とし、FoundationOne CDxは前述のTMBスコアで20mut/Mbを暫定的なカットオフ値としていました。しかし、2020年4月に迅速承認に向けての申請がなされたTMB-highに対するペムブロリズマブの研究ではFoundationOne CDxを用いていますがTMBスコアのカットオフ値を10としています。様々な臨床試験がこのFoundationOne CDxのTMBスコアを元に組まれており、したがって今後出てくるTMB-highに関するエビデンスもこれを根拠としてゆくようになると思われます。となると、この値が今後の業界標準となってゆくかもしれません。
POLEとMSI/MMRとhypermutationの関係
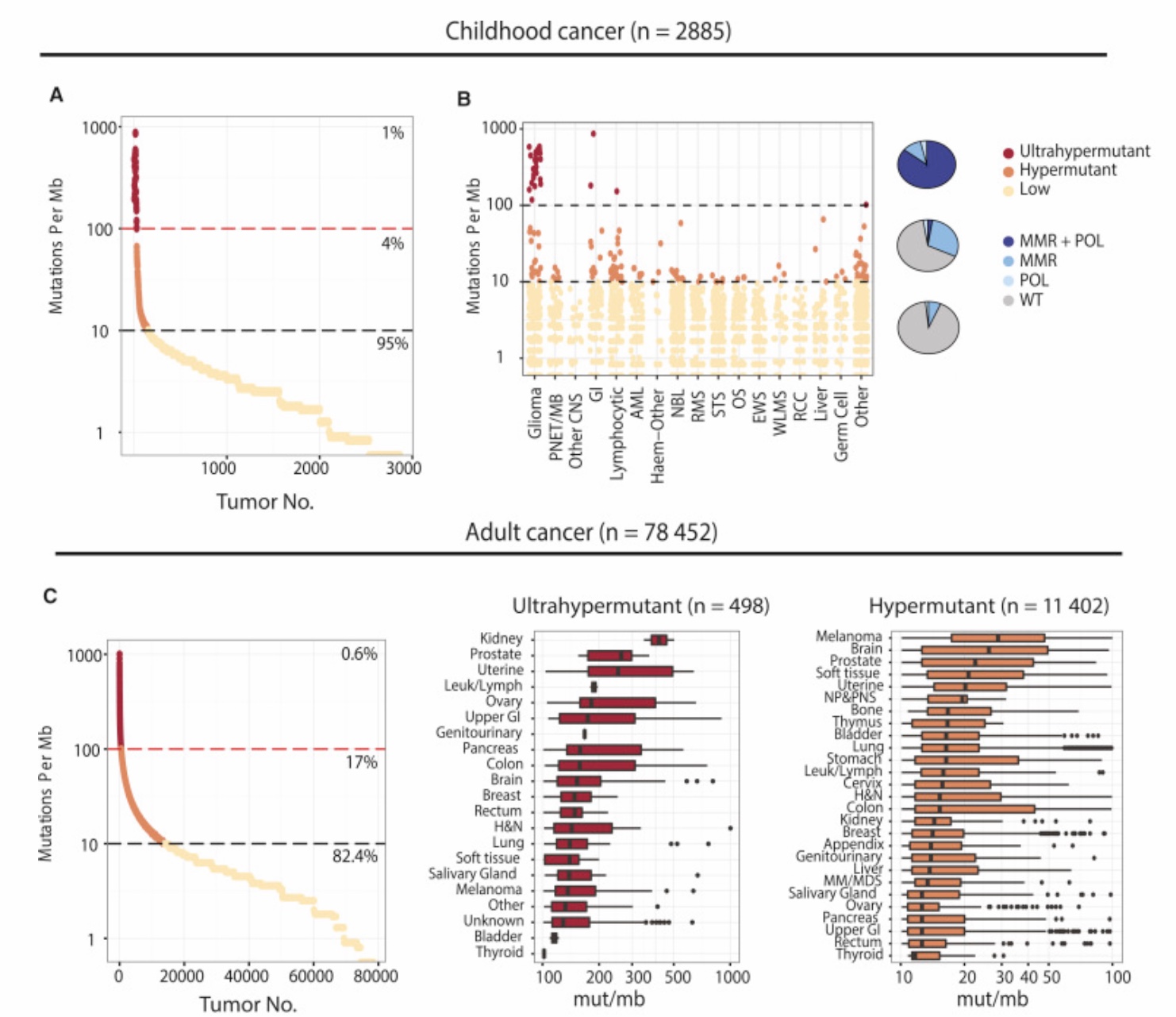
TMBが高いhypermutated固形がんになる要因としてはMSI-HやMMRがよく知られていますが、これらよりもさらに高いTMBになる要因としてPOLEやPOLDが知られています。下記の文献ではTMBが10/Mb以下をlow mutated、10〜100/Mbをhypermutated、100/Mb以上をultrahypermutatedとしてそれぞれの特性を調べていますが、hypermutatedのクラスタにはMSI-Hの関与が大きいですがultrahypermutatedになるとむしろMSI-Hの関与の割合が減ってPOLEまたはPOLE+MMRの重要性が高まることが示唆されています。ultrahypermutatedに該当するのは腎癌・前立腺癌・消化管癌・子宮体癌などがあるほか、小児のgliomaが圧倒的にTMBの高い腫瘍とされています。
We present an extensive assessment of mutation burden through sequencing analysis of >81,000 tumors from pediatric and adult patients, including tumors with hypermutation caused by chemotherapy, carcinogens, or germline alterations. Hypermutation was detected in tumor types not previously associa …
https://www.ncbi.nlm.nih.gov/pubmed/29056344
TMBは免疫チェックポイント阻害剤の治療効果に関連する
悪性腫瘍の総数はTMB(tumor mutation burden)としてしばしばがん遺伝子パネル検査に基づくがんゲノム医療でも議論されますが、その理由はTMBの高さが免疫チェックポイント阻害剤(ICI)の奏効率と相関することが明らかにされたことにより、TMBの高さががん薬物療法のバイオマーカーの一つと認識されるようになったからです。TMB高値はICIの有効性の高さを予測させる因子の一つとは言えそうです。
最初に報告された悪性黒色腫のmutation loadとCTLA-4抗体の有効性の関係
2014
Original Article from The New England Journal of Medicine — Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma
https://www.nejm.org/doi/full/10.1056/NEJMoa1406498
悪性黒色腫のPD-1抗体との関係
2016。BRCA2などにも影響を受けるかもしれない。
High mutational loads are associated with improved survival in melanoma patients but
are not predictive of response to anti-PD-1 therapy, suggesting that other genomic
and non-genomic features also contribute to response patterns on PD-1 checkpoint blockade
therapy.
https://www.cell.com/cell/fulltext/S0092-8674(16)30215-X
非小細胞肺癌でも変異量とICIの感受性に関係

Immune checkpoint inhibitors, which unleash a patient’s own T cells to kill tumors, are revolutionizing cancer treatment. To unravel the genomic determinants of response to this therapy, we used whole-exome sequencing of non–small cell lung cancers treated with pembrolizumab, an antibody targeting programmed cell death-1 (PD-1). In two independent cohorts, higher nonsynonymous mutation burden in tumors was associated with improved objective response, durable clinical benefit, and progression-free survival. Efficacy also correlated with the molecular smoking signature, higher neoantigen burden, and DNA repair pathway mutations; each factor was also associated with mutation burden. In one responder, neoantigen-specific CD8+ T cell responses paralleled tumor regression, suggesting that anti–PD-1 therapy enhances neoantigen-specific T cell reactivity. Our results suggest that the genomic landscape of lung cancers shapes response to anti–PD-1 therapy.
Despite the remarkable success of cancer immunotherapies, many patients do not respond to treatment. Rizvi et al. studied the tumors of patients with non–small-cell lung cancer undergoing immunotherapy. In two independent cohorts, treatment efficacy was associated with a higher number of mutations in the tumors. In one patient, a tumor-specific T cell response paralleled tumor regression.
Science , this issue p. [124][1]
[1]: /lookup/doi/10.1126/science.aaa1348
https://science.sciencemag.org/content/348/6230/124
Original Article from The New England Journal of Medicine — First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer
https://www.nejm.org/doi/10.1056/NEJMoa1613493
イピリムマブ+ニボルマブ併用療法でも。
Original Article from The New England Journal of Medicine — Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden
https://www.nejm.org/doi/full/10.1056/NEJMoa1801946
Hellmann et al. examine non-small-cell lung cancers treated with combined PD-1 and
CTLA-4 blockade using whole-exome sequencing and find that high tumor mutation burden
is the strongest feature associated with improved objective response, durable benefit,
and progression-free survival in multivariable analysis.
https://www.cell.com/cancer-cell/fulltext/S1535-6108(18)30123-5
Hellmann et al. evaluate the impact of tumor mutational burden on the efficacy of
nivolumab monotherapy or combination with ipilimumab in patients with small-cell lung
cancer (SCLC). They show that treatment efficacy and the increased benefit of the
combination are most substantial in SCLC with high tumor mutational burden.
https://www.cell.com/cancer-cell/fulltext/S1535-6108(18)30172-7
非小細胞肺癌に対して。2018
術前化学療法でも
Correspondence from The New England Journal of Medicine — Neoadjuvant PD-1 Blockade in Resectable Lung Cancer
https://www.nejm.org/doi/10.1056/NEJMc1808251
非小細胞肺癌の術前化学療法でも。2018
尿路上皮癌でも
Atezolizumab showed durable activity and good tolerability in this patient population.
Increased levels of PD-L1 expression on immune cells were associated with increased
response. This report is the first to show the association of TCGA subtypes with response
to immune checkpoint inhibition and to show the importance of mutation load as a biomarker
of response to this class of agents in advanced urothelial carcinoma.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)00561-4/fulltext
Atezolizumab showed durable activity and good tolerability in this patient population.
Increased levels of PD-L1 expression on immune cells were associated with increased
response. This report is the first to show the association of TCGA subtypes with response
to immune checkpoint inhibition and to show the importance of mutation load as a biomarker
of response to this class of agents in advanced urothelial carcinoma.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)00561-4/fulltext
TMPとICIの奏効率は多臓器で相関する
https://www.nejm.org/doi/full/10.1056/NEJMc1713444
TMBとPD-L1染色とICIの有効性の関係
Treatment with immune checkpoint blockade (ICB) with agents such as anti-programmed cell death protein 1 (PD-1), anti-programmed death-ligand 1 (PD-L1), and/or anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) can result in impressive response ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6336005/
MSI-H固形がんとペムブロリズマブの機序に関する総説

Immunologic checkpoint blockade with antibodies that target cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and the programmed cell death protein 1 pathway (PD-1/PD-L1) have demonstrated promise in a variety of malignancies. Ipilimumab (CTLA-4) and pembrolizumab (PD-1) are approved by the US Food and Drug Administration for the treatment of advanced melanoma, and additional regulatory approvals are expected across the oncologic spectrum for a variety of other agents that target these pathways. Treatment with both CTLA-4 and PD-1/PD-L1 blockade is associated with a unique pattern of adverse events called immune-related adverse events, and occasionally, unusual kinetics of tumor response are seen. Combination approaches involving CTLA-4 and PD-1/PD-L1 blockade are being investigated to determine whether they enhance the efficacy of either approach alone. Principles learned during the development of CTLA-4 and PD-1/PD-L1 approaches will likely be used as new immunologic checkpoint blocking antibodies begin clinical investigation.
https://ascopubs.org/doi/pdf/10.1200/JCO.2014.59.4358
微小環境との関係
The mechanisms by which immune checkpoint blockade modulates tumor evolution during therapy are unclear. We assessed genomic changes in tumors from 68 patients with advanced melanoma, who progressed on ipilimumab or were ipilimumab-naive, before and after nivolumab initiation (CA209-038 study). Tumo …
https://www.ncbi.nlm.nih.gov/pubmed/29033130
ドライバー遺伝子がある場合はICIの治療効果との相関は薄れる
Natalie I. Vokes JCO 2019
臓器横断的に変異量はICI後の長期予後を予測する
そしてNature Geneticsにはこれに関してMSK-IMPACTのデータを活用して、ICIを受けた1,662人とICIを受けなかった5,371人のデータから、臓器横断的にTMBがICI治療後の長期生存に関連する因子であるということに関するレターが掲載されました。
Analysis of advanced cancer patients treated with immune-checkpoint inhibitors shows that tumor mutational burden, as assessed by targeted next-generation sequencing, predicts survival after immunotherapy across multiple cancer types.
https://www.nature.com/articles/s41588-018-0312-8
ただし、宿主状態によってもICIの効果は大きく左右されるし、PBRM1やARID1Aなど免疫療法の奏効に相関する他の因子も見つかっているので、TMBは数値がいくらならICIが有効とか無効とか一律に数値で決められるパラメータではないことに注意が必要です。
特に、数多くの臨床試験からICIの有効性予測因子としてECOG PSの良さが挙げられていますから、がんゲノム情報だけでなく患者背景など臨床所見を軽視してTMBを特別扱いしてはいけません。
MSI-H固形がんに対する承認薬が今後TMB-high全般に拡大されるかは今後の課題
MSI-H固形がんに対するペムブロリズマブの前向き第2相試験(KEYNOTE-158試験)のエビデンスを根拠にして、MSI-H固形がんに対しては2018年12月からペムブロリズマブが承認されました。

PURPOSE Genomes of tumors that are deficient in DNA mismatch repair (dMMR) have high microsatellite instability (MSI-H) and harbor hundreds to thousands of somatic mutations that encode potential neoantigens. Such tumors are therefore likely to be immunogenic, triggering upregulation of immune checkpoint proteins. Pembrolizumab, an anti‒programmed death-1 monoclonal antibody, has antitumor activity against MSI-H/dMMR cancer. We report data from the phase II KEYNOTE-158 study of pembrolizumab in patients with previously treated, advanced noncolorectal MSI-H/dMMR cancer. PATIENTS AND METHODS Eligible patients with histologically/cytologically confirmed MSI-H/dMMR advanced noncolorectal cancer who experienced failure with prior therapy received pembrolizumab 200 mg once every 3 weeks for 2 years or until disease progression, unacceptable toxicity, or patient withdrawal. Radiologic imaging was performed every 9 weeks for the first year of therapy and every 12 weeks thereafter. The primary end point was objective response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as assessed by independent central radiologic review. RESULTS Among 233 enrolled patients, 27 tumor types were represented, with endometrial, gastric, cholangiocarcinoma, and pancreatic cancers being the most common. Median follow up was 13.4 months. Objective response rate was 34.3% (95% CI, 28.3% to 40.8%). Median progression-free survival was 4.1 months (95% CI, 2.4 to 4.9 months) and median overall survival was 23.5 months (95% CI, 13.5 months to not reached). Treatment-related adverse events occurred in 151 patients (64.8%). Thirty-four patients (14.6%) had grade 3 to 5 treatment-related adverse events. Grade 5 pneumonia occurred in one patient; there were no other treatment-related fatal adverse events. CONCLUSION Our study demonstrates the clinical benefit of anti–programmed death-1 therapy with pembrolizumab among patients with previously treated unresectable or metastatic MSI-H/dMMR noncolorectal cancer. Toxicity was consistent with previous experience of pembrolizumab monotherapy.
https://ascopubs.org/doi/full/10.1200/JCO.19.02105
しかし現時点ではMSI-H固形がんのエビデンスをTMB-high固形がんにそのまま外挿するわけにはいきません。現時点(2019年11月)ではTMB-highの患者群に対するICIの前向き無作為化試験が実施されたわけではないので、MSI-H固形がんに対するペムブロリズマブは国内・FDAとも承認がありエビデンスレベル1Aですが、TMB-highに対するICIはエビデンスレベル3A相当でしょうか。
そして、2020年4月にFDAがTMB-highに対するペムブロリズマブを迅速承認に向けて審査することが発表されました*5。これはESMO2019で発表されたKEYNOTE-158の結果に基づくもののようです*6。なお、KEYNOTE-158は前述のようにMSI-Hに対する臨床試験であるためにTMB-highに対する解析は探索的研究であることと、エントリーされている腫瘍の臓器は比較的免疫チェックポイント阻害剤の有効性を期待しやすいものが多いことに注意が必要です。なお、このときのTMB-highの判定はFoundationOne CDxを用いてTMB score 10をカットオフ値としていたようです。これまではTMB highのカットオフ値は20としていることが多かったので、この辺は今後は修正されてゆく流れになりそうです。
Amazonで角南 久仁子, 畑中 豊, 小山 隆文のがんゲノム医療遺伝子パネル検査実践ガイド。アマゾンならポイント還元本が多数。角南 久仁子, 畑中 豊, 小山 隆文作品ほか、お急ぎ便対象商品は当日お届けも可能。またがんゲノム医療遺伝子パネル検査実践ガイドもアマゾン配送商品なら通常配送無料。
https://amzn.to/3v7ETj8
この記事に対するコメント
このページには、まだコメントはありません。
更新日:2020-04-22 閲覧数:8367 views.